About RTI
With a strong commitment to health, safety, and environmental stewardship, RTI continues to set industry standards, ensuring healthcare workers’ safety and contributing to sustainable workplace environment efforts.
At RTI, we are driven by innovation and a commitment to health, safety, and environmental stewardship. Our mission is to reduce the worldwide spread of infectious diseases by providing safe, reliable medical devices. Over the years, we have expanded our product offerings to include a range of syringes, blood collection devices, and IV catheters, all designed with the same focus on safety and efficiency.
Through innovation, education, and the development of safe and reliable medical devices, Retractable Technologies, Inc. strives to be a catalyst in reducing the worldwide spread of infectious disease.

A Message From Our CEO
In the late 1980’s I was witnessing the deterioration and loss of two dear friends from AIDS. Around the same time, I saw a doctor give an interview regarding needlestick injuries claiming engineers do not care about the safety of healthcare workers. I was moved to action, I visited my local pharmacy to pick up traditional syringes, acquired a grant from the National Institutes of Health (NIH), and have been on a relentless fight to provide clinicians with the safe needle products they need to give them a safe work environment. Eighty percent of America’s leading hospitals now use automated retraction, which is the only safety-engineered technology that effectively reduces needlestick injuries. Unfortunately, in many facilities nurses still blame themselves for needlesticks that occur with poorly engineered devices. There is a simple explanation of why RTI out-designed and out performed the world’s largest and oldest syringe manufacturers. They were designing add-on safety attachments to protect their existing manufacturing equipment, while we, worked with clinicians across the nation to develop a safety product from scratch to truly protect America’s dedicated healthcare workers.
Thanks to the more than 30 years of dedication of some 150 employees in the small town of Little Elm, Texas, RTI is now the world leader in the design and manufacture of effective safety needle products. However, our job is not done until every clinician can go to the cabinet or drawer and grab any product confidently assured that it is designed with safety first and the goal that they will be able to work their entire career without experiencing a single needlestick.
Thomas Shaw | CEO

Humble Beginnings
On May 4, 1994, RTI was incorporated in Texas to design, develop, manufacture, and market medical safety devices for the healthcare industry.

Grand Opening
- 1996 RTI’s corporate office and first building opened its doors
- Consisting of 18,000 square feet of mixed-use manufacturing, warehouse, and office spaces.
- This was the first of many installments for the company’s site.

The VanishPoint® 1997
The 3mL VanishPoint® syringe became commercially available in 1997 and the VanishPoint® blood collection tube holder was awarded “Top of the Line” by Risk & Insurance magazine.

Leo B. Hart Humanitarian Award
RTI’s founder, Thomas Shaw was presented the Leo B. Hart Humanitarian Award from the University of Arizona Alumni Association for his work with RTI to protect medical workers from blood-borne-diseases.

Pioneers
- 1998 the San Francisco Chronicle published a series of articles about HIV, hepatitis, and the needlestick problem.
- July 1, 1999, California’s law requiring the use of safety medical devices went into effect.
- Meanwhile, RTI worked continuously to encourage and assist in the passage of safety needle legislation by individual states and at the federal level.

Needlestick Safety & Prevention Act
When the federal Needlestick Safety and Prevention Act was signed into law in November 2000, RTI’s founder, Thomas Shaw was present at the signing in the Oval Office.

60 Minutes
- February 25, 2001, the CBS news program “60 Minutes” broadcast its lead segment on safety needles and the market obstacles to their widespread use with investigative reporter Mike Wallace who interviewed RTI’s CEO, Thomas J. Shaw, along with needlestick victims.

Awards
RTI won multiple awards from prestigious companies such as:
- Deloitte Technology Fast 500
- Frost and Sullivan Product Quality Leadership of the Year Award
- Texas Crescent Technology Fast 50 Award in 2003
- Metroplex Technology Business Council Tech Titan Fast 50 Award Five Year Category
- Frost and Sullivan Product Quality Leadership of the Year Award

Expansion and Innovations
- By the end of 2005, RTI’s second mixed-use building was completed, consisting of 45,000 square feet.
- As growth continued, in 2009 this building was expanded by an additional 45,000 square feet with 8,600sf devoted to manufacturing, 10,000sf to office space and the remainder becoming warehouse.
- RTI also introduced the VanishPoint® IV catheter product line as RTI’s growth continued.

MTBC Awards
RTI won the following awards from prestigious companies such as:
- Metroplex Technology Business Council Corporate Horizon Award
- Metroplex Technology Business Council Tech Titan Fast 50 Award Five Year Category

Swine Flu
- 2009 H1N1, what became commonly known as the swine flu, exploded onto the world stage.
- In response to the pandemic RTI was awarded a contract by US Department of Health and Human Services to support the United States’ efforts to vaccinate the population against H1N1.
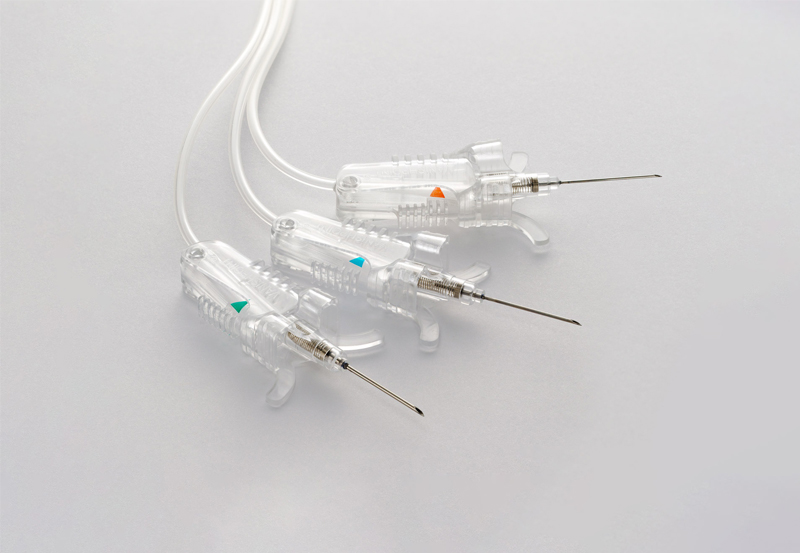
One Invention After Another
- The VanishPoint® blood collection set was introduced in 2011.
- The EasyPoint® needle became commercially available in 2014.

COVID-19 Pandemic
- In response to the COVID-19 pandemic in June 2020, RTI began providing VanishPoint safety syringes to the U.S. Government in support of the vaccination effort
- From 2020-2022 RTI provided in excess of 1.2 billion units to combat COVID-19 worldwide.
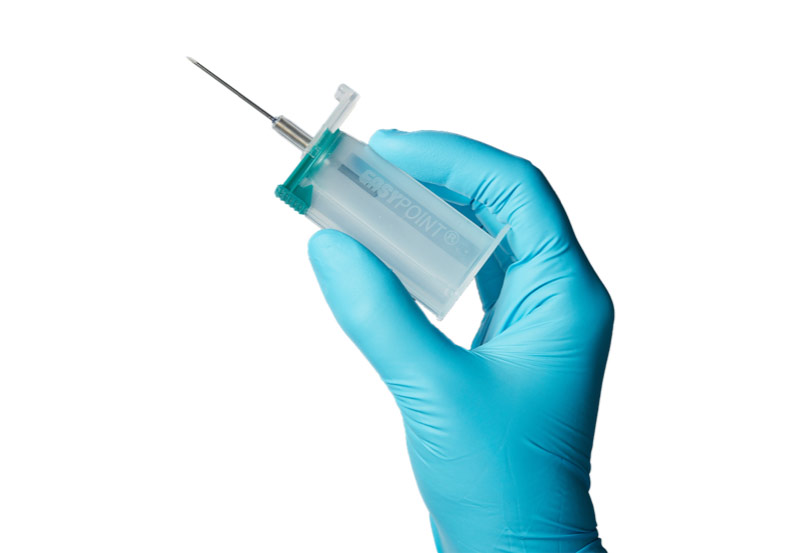
Our Latest Product
In 2021, the EasyPoint® blood collection tube holder with needle was brought to market, this is RTI’s most recent invention, but not the last. RTI continues to search for new ways to ensure safety for healthcare workers.

New Expansion
- In June 2022, RTI completed construction on its third mixed use building consisting of 55,000 square feet of warehouse and training facilities.
- By the end of 2023, the finishing touches of an expansion on the original building was completed.

Still on the Mission
Today RTI employs over 200 people, with twenty percent of those employees crossing the twenty-year threshold at RTI. Throughout its history, RTI continues to make the necessary inroads to establish itself as the market leader in safety syringe technology.





American Excellence, Vision, & Innovation
RTI is the World Leader in the Design and Manufacture of Effective Safety Needle Products
1.2 Billion
for COVID-19 Immunizations.
8 of 10
U.S. hospitals* use VanishPoint Technology
20,000+
their nurses with our technology.





RTI has secured significant contracts with the U.S. government, particularly with the Department of Health and Human Services (DHHS). Notably, we provided VanishPoint syringes during the H1N1 influenza pandemic in 2009 and again during the COVID-19 pandemic. These contracts highlight our ability to meet the rigorous standards and large-scale demands required by federal agencies.
RTI is focused on reducing healthcare related exposure to bloodborne pathogens. Our solutions are based on engineering controls—devices that effectively reduce the risk of exposure associated with needlestick injury and device reuse. Unfortunately, healthcare worker access to innovative products is often limited by contracts and entrenched market interests. RTI has battled—and continues to battle—to provide safe, innovative products to healthcare workers.
From recycling the excess plastic our production makes, to beehives on our campus, to incorporating environmental efficiency, we make sustainability part of our daily lives.

To build a model corporation for employees, customers, community, and shareholders based on performance, pride, and profit; To set a standard for excellence, effectiveness, and integrity in selling and delivering our products; and to establish mutually beneficial relationships with other companies who share our overall vision.